For HCPs only.
Cytotect CP® is indicated for prophylaxis of clinical manifestations of cytomegalovirus infection in patients subjected to immunosuppressive therapy, particularly transplant recipients.1
The concomitant use of adequate virostatic agents should be considered for CMV prophylaxis.1 Basic Information available here.
Amplify your CMV control
When targeting CMV following transplantation, watch how an ensemble approach can amplify prophylaxis in immunosuppressed patients.

- Remains a major cause of morbidity and mortality in transplant patients2–4
- Is associated with significantly decreased survival in solid organ transplant5
- Is associated with an increased risk of organ rejection2,6,7
Limitations of the virostatic mode of action
- Antivirals block viral replication, but are unable to destroy intra- or extracellular viruses, which may result in a rebound effect when antiviral therapy is discontinued2,8,9
- Antiviral drugs are unable to prevent infection of other cells or organs by free virus particles9


Cytotect CP® prevents cell penetration of the virus by binding CMV surface antigens1,10

Cytotect CP® labels CMV and presents it for phagocytosis (internalization and destruction by immune cells)1,10

Cytotect CP® can activate CMV-reactive immune cells for long-lasting CMV-specific immune responses1,10

Cytotect CP® inhibits pro-inflammatory cells, decreasing cytokine production and immune-mediated damage1,6,10
Go backstage to learn more about Cytotect CP®'s mode of action
Prevention of CMV infection and disease
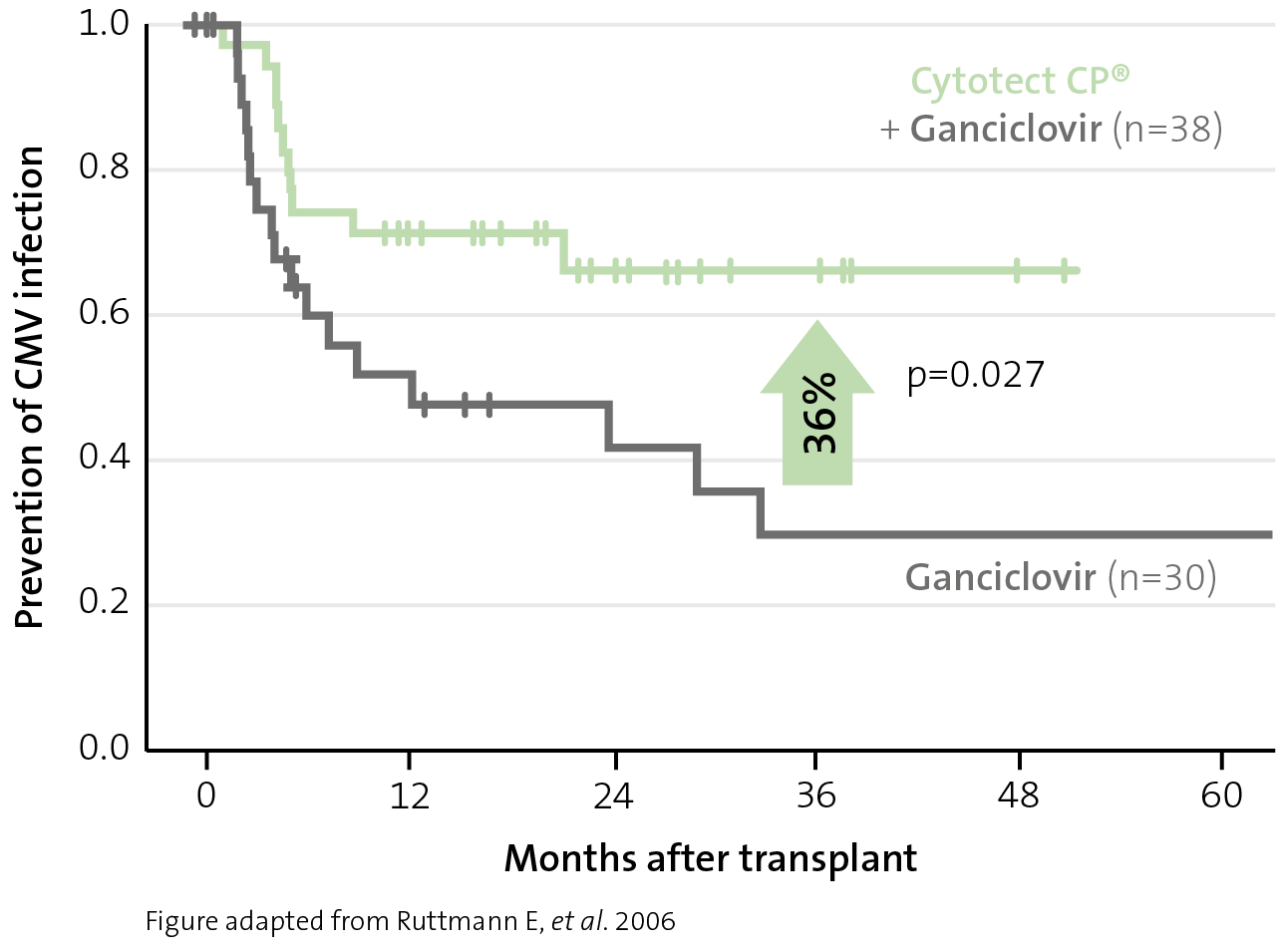
reduction in CMV infection
with Cytotect CP® combination prophylaxis in lung transplant patients versus antiviral monotherapy11
reduction in CMV disease with Cytotect CP® combination prophylaxis in heart transplant patients versus monotherapy with Cytotect CP®12
Prevention of organ rejection
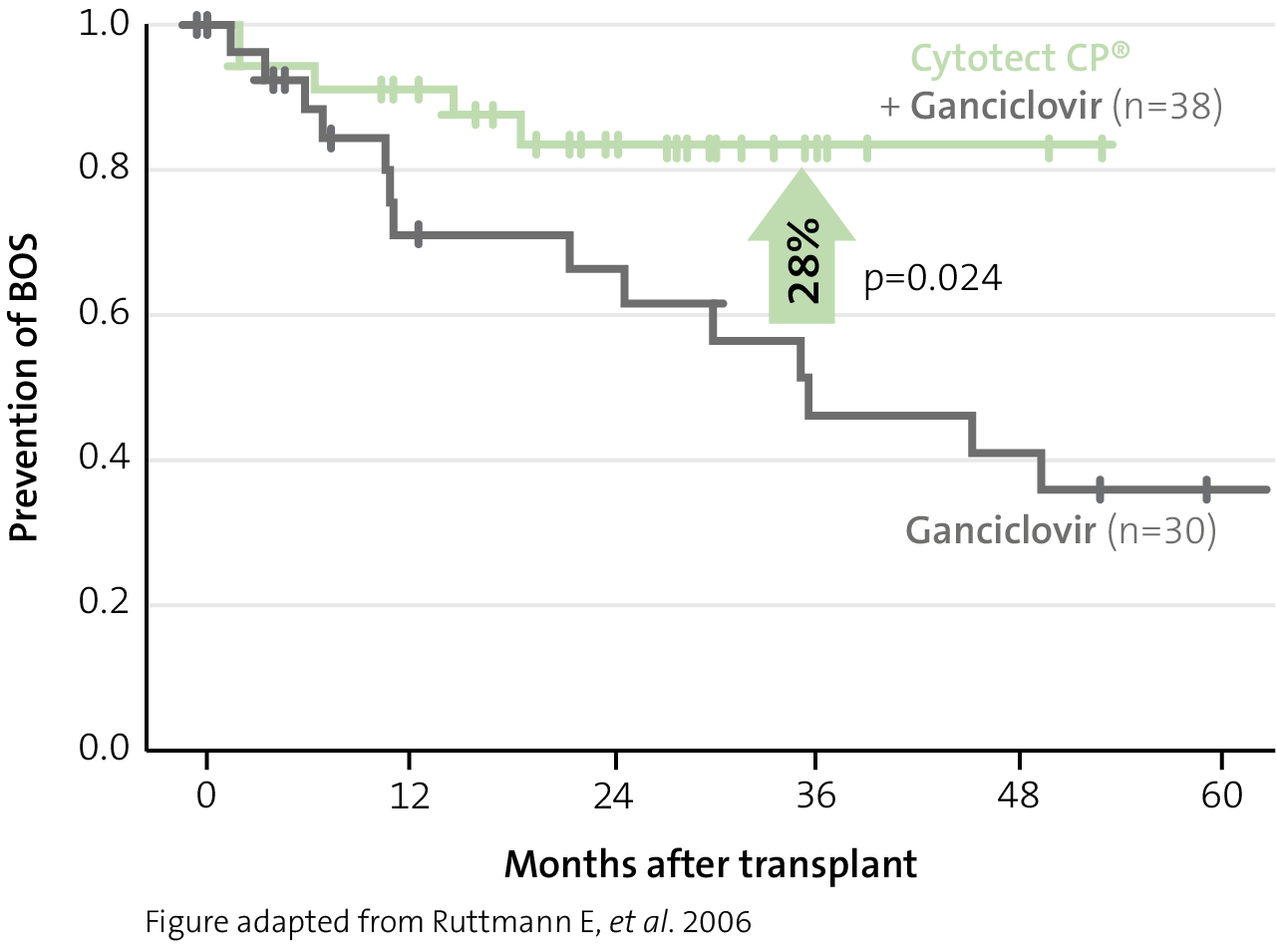
reduction in BOS
(bronchiolitis obliterans syndrome) with Cytotect CP® combination prophylaxis in lung transplant patients11
relative risk reduction for CAV (cardiac allograft vasculopathy) with Cytotect CP® combination prophylaxis in heart transplant patients (ARR: 6.4%)12
Improvement of survival
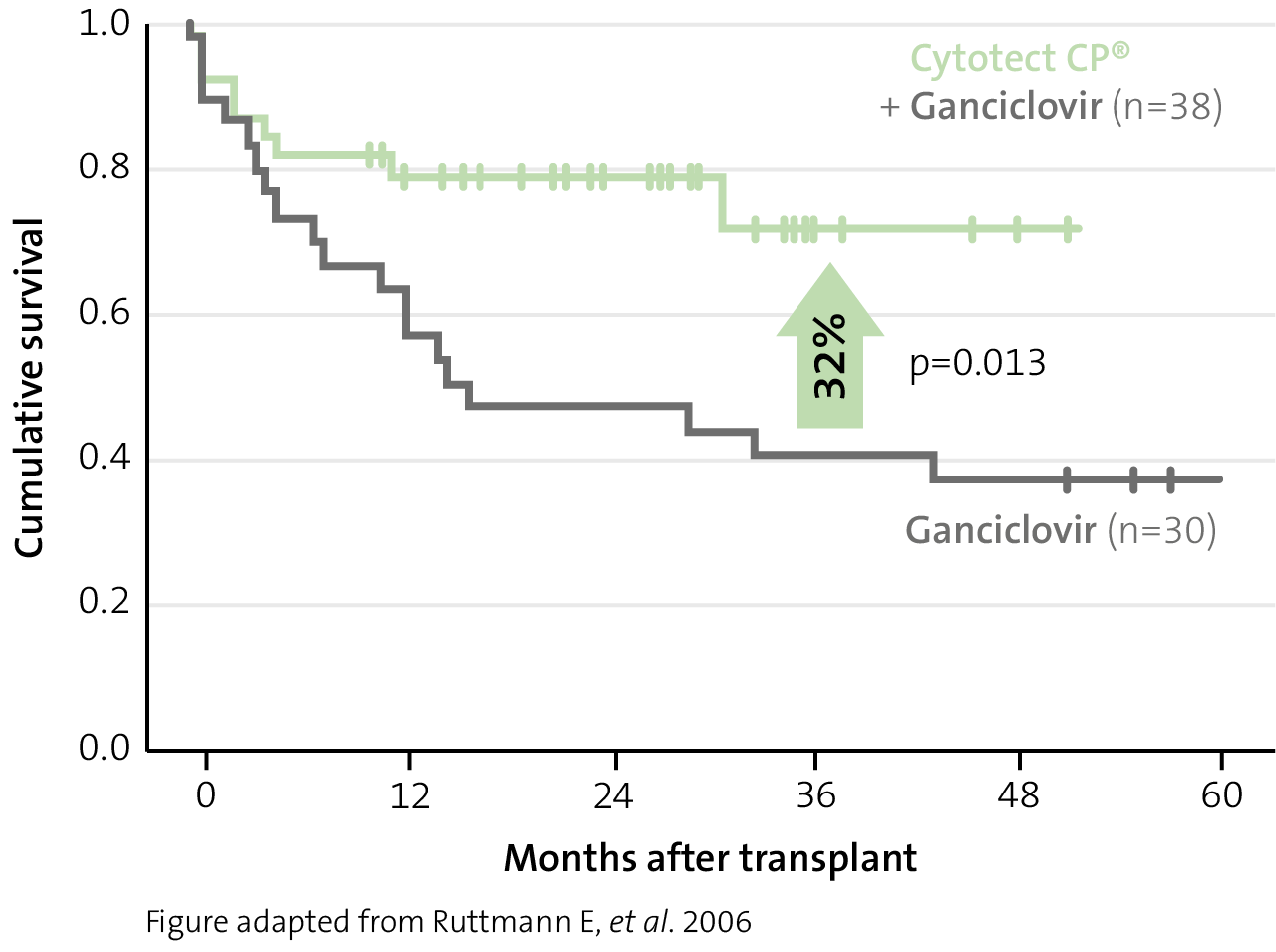
improvement in 3-year survival with Cytotect CP® combination prophylaxis in lung transplant patients versus antiviral monotherapy11
Cytotect CP®:
Cytotect CP® has shown favorable tolerability in clinical trials1 and in RWE10
For more information, please refer to the most recent Summary of Product Characteristics.
- Cytotect CP® Biotest. Summary of Product Characteristics.
- Snydman DR, et al. Transplant Proc. 2011;43(3 Suppl):S1–17.
- Ljungman P, et al. Hematol Oncol Clin North Am. 2011;25(1):151–69.
- Walker CM, et al. Biol Blood Marrow Transplant. 2007;13(9):1106–15.
- Desai R, et al. Transplantation. 2015;99(9):1989–94.
- Kornberg A, World J Hepatol. 2015;7(11):1494–508.
- Preiksaitis JK, et al. Am J Transplant. 2005;5(2):218–27.
- Fishman JA, Am J Transplant. 2017;17(4):856–879.
- Andreoni KA, et al. J Med Virol. 2002;67(1):33–40.
- Grossi P, et al. Transplantation. 2016;100(Suppl 3):S1–4.
- Ruttmann E, et al. Transplantation. 2006;81(10):1415–20.
- Bonaros NE, et al. Transplantation. 2004;77(6):890–897.
- Lachmann R, et al. PLoS one. 2018;13(7):e0200267.
- Adland E, et al. Front Microbiol. 2015;6:1016.
Copyright © 2024 Biotest AG. All rights reserved. Biotest AG is proprietor of all trademarks and tradenames used on this website.